According to the US Food and Drug Administration, Orthovisc’s four-injection therapy significantly improves knee osteoarthritis compared to saline and arthrocentesis. The clinical study underscores Orthovisc’s potential for long-lasting relief and enhanced patient mobility after a single cycle.
Orthovisc knee injections provide a non-surgical alternative for those who are not yet suitable candidates for total knee replacement surgeries but require symptomatic relief. Medical professionals must administer this treatment to ensure patients a safe and effective outcome for their knee osteoarthritis (OA) concerns. Adherence to the correct usage guidelines ensures a seamless medical procedure.
In this article, we will discuss Orthovisc’s indications, recommended dosage and administration, and potential risks.
Key Takeaways
- The US Food and Drug Administration has approved Orthovisc for treating knee osteoarthritis (OA) in patients who have failed to respond to conservative therapies and simple analgesics.
- The manufacturer of Orthovisc, in conjunction with the US Food and Drug Administration, has established specific indications for the injection treatment and a set of contraindications that medical professionals must strictly adhere to.
- Following the recommended Orthovisc dosage and administration can help medical professionals ensure a smooth-flowing treatment procedure for their patients.
- Orthovisc has specific precautions, associated warnings, and potential adverse reactions like other medical or aesthetic procedures.
About: Medica Depot is your trusted all-in-one supplier, offering a range of high-quality medical injectables and supplies. If you’re looking to buy Orthovisc online, you can do so on Medica Depot quickly and easily. We offer a worry-free experience in searching for the best and most popular products on the market. Whether for health professionals, plastic surgeons, dermatologists, licensed estheticians, or other specialists, we can offer genuine, brand-name products you may need. With Medica Depot, we prioritize serving you better to improve the patient’s quality of life.
Orthovisc Indications and Usage

Orthovisc knee injection is a viscosupplementation product that can treat knee pain caused by osteoarthritis (OA). This therapy supplement the lost hyaluronic acid (HA) in the joint’s synovial fluid. The fluid surrounds the joints, acting as a lubricant and shock absorber for the area.
The US Food and Drug Administration has approved Orthovisc for treating knee osteoarthritis (OA) in patients who have failed to respond to conservative therapies and simple analgesics. Medical professionals in the United States and Canada can utilize Orthovisc solely for the knee joints.
Clinical studies have demonstrated the safety and efficacy of Orthovisc in providing pain relief for several months. According to the American Association of Orthopedic Surgeons, the most recent research has not found viscosupplementation effective at significantly reducing or improving function; however, some patients have reported receiving pain relief and better quality of life.
Contraindications and Special Populations

While the Orthovisc manufacturer and the US Food and Drug Administration added specific indications for the injection treatment, various contraindications for medical professionals to strictly follow also exist. Understanding these contraindications can help providers prevent further complications that may compromise the patient’s health.
- Do not administer to patients with hypersensitivity to hyaluronate formulations or gram-positive bacterial proteins
- Do not administer to patients with allergies to avian or avian-derived products
- Do not administer Orthovisc in the injection site with skin disease or infection
Furthermore, medical professionals may need to adjust Orthovisc knee injections depending on various special considerations for several populations.
- Elderly Patients: This special population requires more careful monitoring due to potential age-related changes. Medical professionals can adjust the dosage and frequency of Orthovisc knee injections based on the patient’s needs and goals.
- Patients with Comorbidities: These individuals may need special attention due to potential interactions between Orthovisc and other medications. Healthcare providers must thoroughly assess these patients to provide severe risks.
- Athletes: While most athletes may be deemed fit and healthy, they must avoid strenuous or high-impact activities after receiving their Orthovisc knee injections. This helps to ensure the treatment’s effectiveness and prevent potential complications. Moreover, they can ask their doctors for a specific time to return to active lifestyles.
Dosing and Administration
Healthcare providers can help patients decide what treatment suits them and their needs. They can explain Orthovisc vs Monovisc and give patients comprehensive information about these viscosupplements. Moreover, they can also create a tailored treatment plan for their patients, considering the product’s benefits and the patient’s needs, goals, osteoarthritis condition, and overall health.
Furthermore, following the recommended Orthovisc dosage and administration can help medical professionals ensure a smooth-flowing treatment procedure for their patients. This also lessens the potential risks and complications during or after the shots.
- Recommended Dosing Regimens: An Orthovisc knee injection cycle typically requires an adult dose of 2 mL, with three to four injections needed. Medical professionals should give these shots weekly.
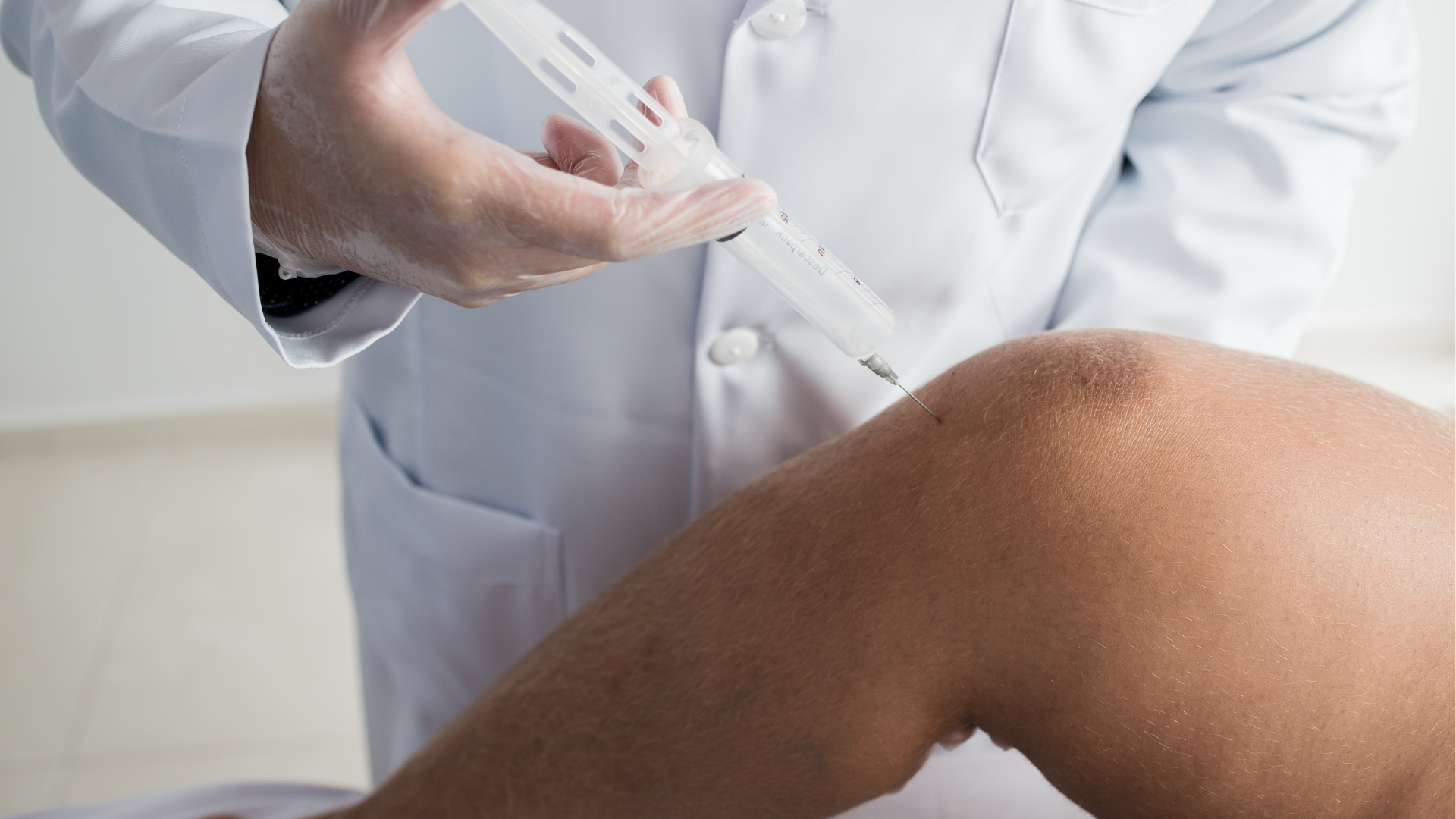
The proper administration technique requires medical professionals to use Orthovisc as an intra-articular injection. These professionals should inject Orthovisc directly into the affected knee joint and use an aseptic technique.
Before injecting, these providers should also remove joint effusion in the injection site to avoid severe risks. Applying local anesthetic can lessen the patient’s discomfort and improve their satisfaction during and after the procedure.
Following the recommended and proper injection technique is crucial for the effectiveness of Orthovisc treatment. Only medical professionals should administer these knee injections to ensure strict adherence to delivering the medication to the affected joint. Incorrect administration may lead to complications and reduced Orthovisc efficacy.
Precautions, Warnings, and Adverse Reactions

Orthovisc has specific precautions, associated warnings, and potential adverse reactions like other medical or aesthetic procedures. However, with a patient-centric approach from medical professionals, their expertise and knowledge can educate patients about this Orthovisc information before the procedure.
Avoiding patients with conditions under the product’s contraindications can significantly help patients prevent health complications. In addition, the Orthovisc manufacturer has yet to test the injection’s safety and efficacy on pregnant women, breastfeeding women, and children under 21.
- The contents of the pre-filled syringe should be used immediately after opening.
- Do not use Orthovisc knee injections if the package has been opened or damaged.
- Store Orthovisc in its original package at room temperature (below 77°F/250C).
- Do not use disinfectants containing quaternary ammonium salts for skin preparation, as hyaluronic acid can precipitate.
Patients can expect common side effects after Orthovisc knee injections. These are temporary and may subside when the body adapts to the medicine. These side effects include pain, swelling, heat, rash, itching, bruising, and redness.
An Orthovisc clinical trial revealed several adverse events that patients reported after the treatment. If these persist or you have other worrisome symptoms, it’s best to contact your healthcare providers immediately. They can help individuals manage these symptoms to avoid health compromise.
- Arthralgia
- Back Pain
- Headache
Conclusion
Orthovisc is indicated for the treatment of knee pain in patients with osteoarthritis who have failed to respond adequately to conservative therapies and simple analgesics. Providers should not use their contraindications to avoid complications. The recommended dosing regimen involves one weekly injection for three weeks, strictly following aseptic techniques to prevent infection.
Patients and healthcare providers must be aware of potential adverse reactions, including pain, swelling, and effusion at the injection site. Following the prescribed guidelines for the safe use of Orthovisc is crucial to minimize risks and ensure optimal therapeutic outcomes for patients suffering from osteoarthritis-related knee pain.
FAQs
1) What is Orthovisc used for?
Orthovisc is a treatment for knee osteoarthritis that causes joint pain and stiffness. The manufacturer designed Orthovisc to allow its injection into the knee joint to relieve pain and improve knee function.
2) How is Orthovisc administered?
Healthcare providers can administer Orthovisc through three to four injections into the knee joint weekly for three to four weeks. The healthcare provider will determine the appropriate dosage and injection schedule based on the patient’s condition.
3) What are the common side effects of Orthovisc?
Common side effects of Orthovisc include pain or swelling at the injection site, warmth or redness around the knee joint, and stiffness or limited range of motion in the knee. These side effects are typically mild and go away within a few days.
4) Is Orthovisc safe?
Orthovisc is generally safe and well-tolerated by patients. However, as with any medical treatment, there is a risk of side effects. Patients should discuss their medical history and concerns with their healthcare provider before starting Orthovisc treatment.
References
- ORTHOVISC® High Molecular Weight Hyaluronan. (n.d.). accessdata.fda.gov. https://www.accessdata.fda.gov/cdrh_docs/pdf3/P030019c.pdf
- Patient Information ORTHOVISC® High Molecular Weight Hyaluronan. (n.d.). US Food and Drug Administration. Retrieved April 22, 2024, from https://www.accessdata.fda.gov/cdrh_docs/pdf3/p030019d.pdf