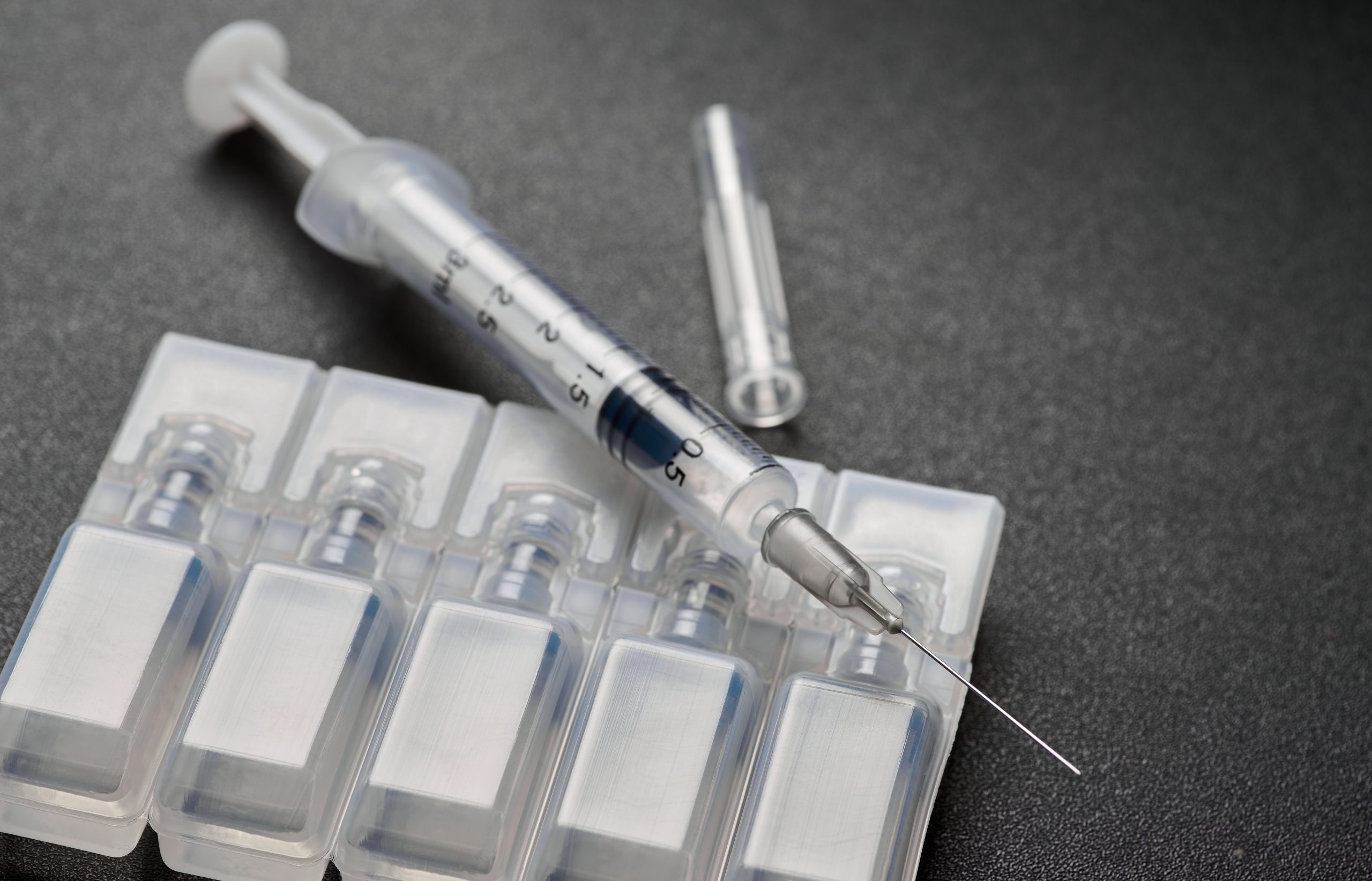
According to the US Food and Drug Administration (FDA), their clinical trial revealed that Orthovisc brings significant pain relief to patients who have not responded to first-line therapies. The study emphasizes the safety and efficacy of Orthovisc to patients, with no considerable reports of adverse events occurrences.
The US FDA’s clinical study supports Orthovisc as a non-surgical solution for patients with knee osteoarthritis. Individuals who are yet to be candidates for knee replacement surgeries and those with early to severe knee OA may opt for Orthovisc’s multiple injection therapy for symptomatic relief and improved joint functionality.
This article will discuss the proper Orthovisc storage, factors influencing product stability and efficacy, and best practices to ensure Orthovisc’s quality.
Key Takeaways
- Orthovisc knee injection aims to restore hyaluronic acid (HA) to the joint’s synovial fluid, enabling reduced friction and pain-free movement.
- Proper storage is crucial for preserving product quality, reducing waste, and optimizing Orthovisc’s efficiency.
- This injection must be stored at room temperature below 77°F (25°C) and avoid freezing the solution.
- Healthcare providers can use Orthovisc up to two years from the date of manufacture, provided it has been stored correctly and has no physical damage or chemical contamination.
- Maintaining the quality of Orthovisc involves adhering to storage guidelines and handling procedures.
About: Medica Depot is your trusted all-in-one supplier, offering a range of high-quality medical injectables and supplies. If you’re looking to order Orthovisc online, you can do so on Medica Depot quickly and easily. We offer a worry-free experience in searching for the best and most popular products on the market. Whether for health professionals, plastic surgeons, dermatologists, licensed estheticians, or other specialists, we can offer genuine, brand-name products you may need. With Medica Depot, we prioritize serving you better to improve the patient’s quality of life.
Understanding Orthovisc Product Stability

Orthovisc capitalizes on its high molecular weight of hyaluronic acid to deliver symptomatic relief to patients with osteoarthritis (OA). Orthovisc shoulder or knee injections are a sterile, non-pyrogenic, viscoelastic solution in a single-use syringe. It aims to restore hyaluronic acid (HA) to the joint’s synovial fluid, enabling reduced friction and pain-free movement.
Medical professionals must understand Orthovisc’s product stability. Various factors may affect the medicine’s stability, influencing Orthovisc’s efficacy and safety.
- Temperature: Medical professionals should not freeze Orthovisc knee injections and should keep them at room temperature in their original packaging. Temperature changes can alter the properties of the medicine.
- Humidity: High humidity can cause products to absorb excess moisture in the air, which can compromise the potency and effectiveness of Orthovisc. Moreover, it can also lead to degradation or even toxicity in some products.
- pH Level: The pH of the solution can also influence the decomposition rate of most drugs, including Orthovisc knee injections. Variations in pH can impact the consistency and shelf-life of products.
- Light Exposure: UV light can initiate chemical reactions that lead to photodegradation. Light induces the interaction between the compound molecules, forming a new compound known as impurity.
Proper storage is crucial for preserving product quality, reducing waste, and optimizing Orthovisc’s efficiency. Maintaining the required storage temperature and sanitary conditions can prevent contamination, physical degradation, and damage.
Effective storage methods ensure that Orthovisc is well-preserved and can save time and money in the long run. By adhering to proper Orthovisc storage, medical professionals can ensure that the medicine’s potency and effectiveness are intact.
Storage Requirements for Orthovisc Injections

Orthovisc knee injections treat pain, stiffness, and swelling in the affected joint. Providers or clinics must store them in their original packaging. The Orthovisc manufacturer recommends that a healthcare provider administer the medication in a clinic, so people typically don’t store it in a household.
- Recommended Temperature and Storage Condition: Professionals must keep the injection at room temperature below 77°F (25°C). According to the US Food and Drug Administration for Orthovisc High-Molecular-Weight Hyaluronan, medical professionals should avoid freezing the solution. Moreover, they cannot and should not resterilize Orthovisc.
- Avoiding Exposure to Light and Extreme Temperatures: It’s generally advisable to avoid such conditions to maintain the integrity of most medications, including Orthovisc knee injections. Extreme temperatures can potentially alter the chemical composition and efficacy of Orthovisc. Similarly, certain medications may degrade when exposed to light.
Shelf Life of Orthovisc Injections

The typical shelf life of Orthovisc knee injections is approximately 24 months, provided professionals adhere to proper storage conditions. Healthcare providers can use Orthovisc up to two years from the date of manufacture, provided it has been stored correctly and has no physical damage or chemical contamination.
- Determining the Expiration Date: Medical professionals can see the Orthovisc expiration dates on the product packaging. It’s essential to check this date to ensure it is within its shelf life and can deliver high-quality safety and efficacy.
Similar to the factors influencing product stability, they can also affect shelf life and product efficacy. These include pepper Orthovisc storage conditions, original and sealed packaging, light exposure, and temperature.
Medical professionals must quickly use Orthovisc knee injections after opening to maintain their freshness and efficacy. They must administer the contents of the syringe immediately and discard any unused Orthovisc.
Best Practices for Orthovisc Storage
Medical professionals understand that proper medicine storage can affect a product’s integrity, safety, and effectiveness in every treatment. For Orthovisc knee injections, they must ensure that the original packaging has not been opened or damaged. This also assures individuals that the Orthovisc came from the manufacturer and is authentic.
Keep Orthovisc injections at room temperature below 77°F (25°C) and avoid freezing them. When storing and handling the product, you must ensure proper sanitary conditions. Furthermore, they should receive appropriate training on good storage practices, regulations, procedures, and safety.
Maintaining the quality of Orthovisc involves adhering to storage guidelines and handling procedures. It’s essential to protect the product from freezing and allow it to reach room temperature approximately 20-45 minutes before use.
Medical professionals use quality management, assurance, and control to pursue a product’s excellence. Medical professionals may purchase at Medica Depot to ensure they receive genuine Orthovisc knee injections, delivering safe and effective knee osteoarthritis treatment to their patients.
Conclusion
Orthovisc is a significant non-surgical treatment for knee osteoarthritis, characterized by joint pain, stiffness, and swelling. Understanding Orthovisc storage conditions can help medical professionals ensure the medicine’s stability, effectiveness, and safety. Keeping Orthovisc at room temperature below 77°F (25°C) is essential.
Adhering to the best practices for Orthovisc storage is crucial in preserving the product’s quality and integrity. Proper handling and storage procedures play significant roles in safeguarding Orthovisc’s efficacy. By following these guidelines, we can ensure that Orthovisc remains effective and safe.
FAQs
1. What is Orthovisc, and what is it used for?
Orthovisc is a non-surgical solution for patients suffering from knee osteoarthritis. It is a multiple injection therapy that aims to restore hyaluronic acid to the joint’s synovial fluid, enabling reduced friction and pain-free movement.
2. What are the proper storage requirements for Orthovisc injections?
Healthcare providers must store Orthovisc injections at room temperature below 77°F (25°C) and should not be frozen. Exposure to light and extreme temperatures should be avoided to maintain the integrity of the product.
3. How long is the shelf life of Orthovisc injections?
The typical shelf life of Orthovisc knee injections is approximately 24 months, provided proper storage conditions are followed. Medical professionals can check the expiration date on the product packaging to ensure the product is within its shelf life and can deliver high-quality safety and efficacy.
References
- SUMMARY OF SAFETY AND EFFECTIVENESS DATA. (n.d.). Retrieved April 25, 2024, from https://www.accessdata.fda.gov/cdrh_docs/pdf3/P030019b.pdf
- ORTHOVISC® High Molecular Weight Hyaluronan. (n.d.). Accessdata.fda.gov. https://www.accessdata.fda.gov/cdrh_docs/pdf3/P030019c.pdf