According to Neustadt et al. (2005), Orthovisc’s high molecular weight hyaluronan composition indicates safety and effectiveness in treating mild to severe cases. This underscores its potential as a promising advancement in non-surgical knee osteoarthritis management, providing significant pain relief to patients.
Knee osteoarthritis (OA) is a prevalent condition causing significant joint pain, stiffness, and swelling. Orthovisc, as a non-surgical solution, offers an efficient solution that transforms patients’ quality of life and knee OA management. This high molecular weight injection’s potential effectiveness opens doors for further exploration and application.
This article will discuss Orthovisc knee injections, their production method, control standards, and potential innovations.
Key Takeaways
- Healthcare professionals may suggest Orthovisc knee injections when conservative therapies have not relieved the patients adequately.
- During the Orthovisc knee injection manufacturing, they may have used automation, computation, software, sensing, networking, advanced facilities, and technologies.
- Anika Therapeutics Inc. has demonstrated its adherence to stringent regulatory standards through successful clinical trials and regulatory approvals.
- Spearheaded by Anika in research and development efforts, they focus on addressing knee osteoarthritis (OA) management, regenerative solutions, arthrosurface joint solutions, and sports medicine.
About: Medica Depot is your trusted all-in-one supplier, offering a range of high-quality medical injectables and supplies. If you’re looking to buy Orthovisc wholesale, you can do so on Medica Depot quickly and easily. We offer a worry-free experience in searching for the best and most popular products on the market. Whether for health professionals, plastic surgeons, dermatologists, licensed estheticians, or other specialists, we can offer genuine, brand-name products you may need. With Medica Depot, we prioritize serving you better to improve the patient’s quality of life.
Overview of Orthovisc Manufacturer

Orthovisc is a non-surgical solution that effectively treats knee osteoarthritis (OA). Its manufacturer is Anika Therapeutics Inc., a Massachusetts-based medical technology company that earned the US Food and Drug Administration approval for Orthovisc in 2004.
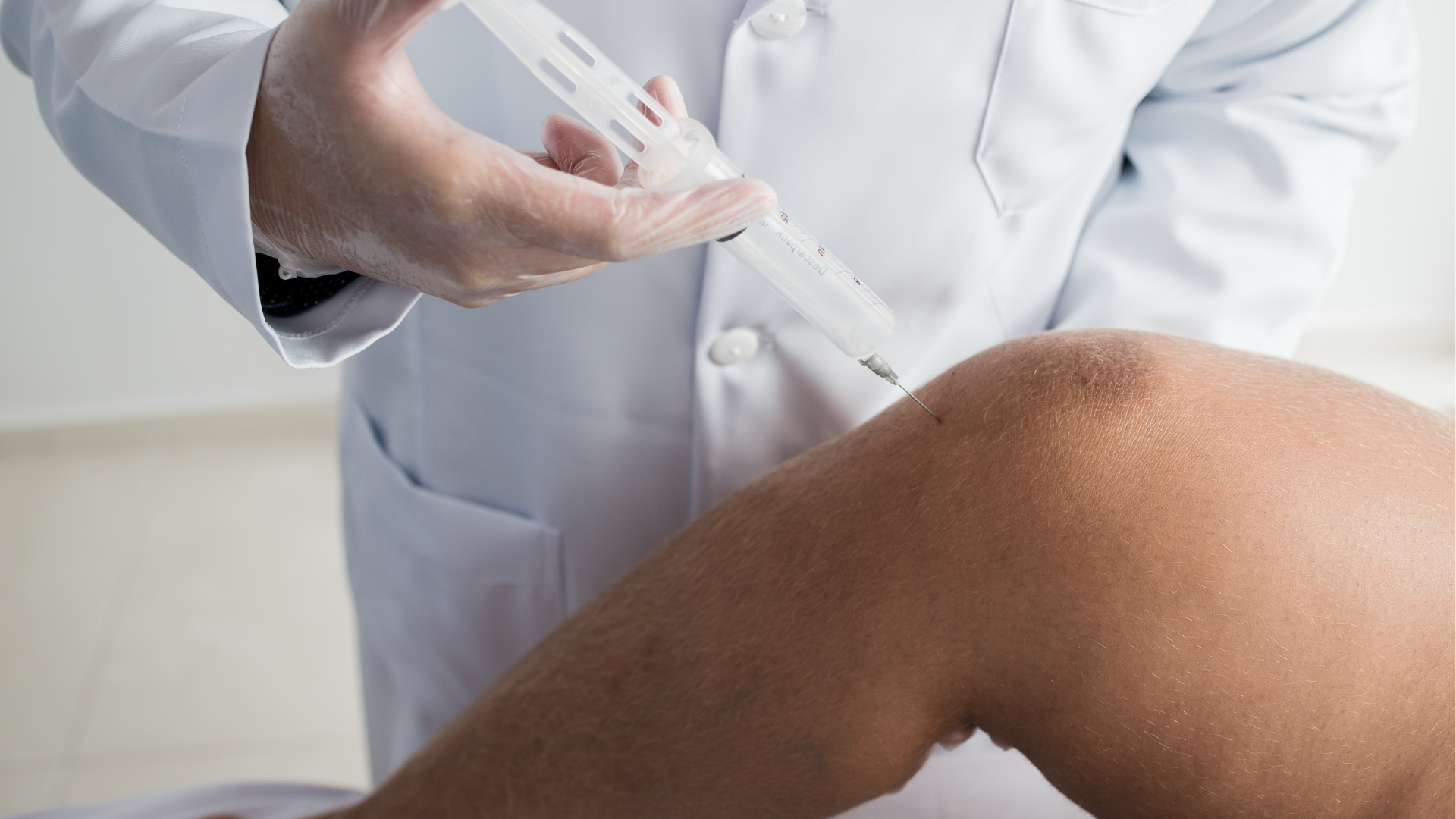
Orthovisc primarily relies on the potency of high-molecular-weight hyaluronic acid (HA) in its injections. This substance mimics the HA found in the joint’s synovial fluid, supplementing the lost HA in the affected area. When injected, Orthovisc acts as a lubricant and shock absorber for the joints, allowing pain-free movement and improved function and mobility.
Healthcare professionals may suggest Orthovisc knee injections when conservative therapies have not adequately relieved the patients. This therapy typically recommends a series of three to four injections, given once weekly. Moreover, understanding the Orthovisc manufacturer can also build trust between them, providers, and patients.
- Ensuring Product Safety and Quality
- Crucial in Understanding Product Development
- Building Trust and Confidence in Orthovisc’s Efficacy
Manufacturing Facility and Production Methods

Anika Therapeutics Inc. is a global company that aims to restore patients’ active lifestyles. The manufacturing process involves creating a sterile, non-pyrogenic, viscoelastic solution in a single-use syringe. This process ensures high hyaluronic acid (HA) content to relieve symptomatic knee osteoarthritis.
State-of-the-art facilities use advanced technology to build products more efficiently, safely, and with higher quality. While limited online resources discuss Anika Therapeutics Inc.’s facilities, it’s worth noting that they potentially utilize smart devices, systems to monitor manufacturing autonomously, and machines to gather and analyze manufacturing data continuously.
Advanced manufacturing technology integrates manufacturing and business activities to create a more efficient operation. During the Orthovisc knee injection manufacturing, they may have used automation, computation, software, sensing, and networking. Moreover, Anika likely used advanced methods and technologies to create its vast array of products.
Regulatory Compliance Standards
With Orthovisc’s safety and efficacy, Anika Therapeutics Inc. inevitably adheres to strict regulatory compliance standards. FDA approval ensures Orthovisc knee injections’ safety, efficacy, and quality before they reach medical professionals and patients.
Anika Therapeutics Inc. has demonstrated its adherence to stringent regulatory standards through successful clinical trials and regulatory approvals. They offer healthcare providers and patients the best quality management of knee osteoarthritis symptoms by strictly following necessary standards.
Orthovisc knee injections involve quality control measures, including monitoring, evaluating, and maintaining the quality of the medicine. These measures aim to identify and correct deviations from established quality standards. This systematic process can minimize defects, lower costs, and enhance customer satisfaction.
Research and Development Efforts

Orthovisc knee injections offer a promising non-surgical solution to individuals who have not gained relief through conservative therapies and simple analgesics. Spearheaded by Anika in research and development efforts, they focus on addressing knee osteoarthritis (OA) management, regenerative solutions, arthrosurface joint solutions, and sports medicine.
Anika has actively engaged in ongoing research initiatives, including Orthovisc-T. This injectable won its CE mark approval for relieving pain and restoring damaged tendon functions caused by chronic injury. Orthovisc-T has yet to receive approval in the United States. Nonetheless, this shows Anika’s commitment to enhancing patients’ lives with degenerative orthopedic diseases.
Anika offers two products for managing knee OA symptoms: Orthovisc and Monovisc. According to Orthovisc’s prescribing information, these injections require three to four shots for optimal outcomes. Meanwhile, Anika also produced Monovisc as their single-injection therapy for knee OA management. Both treatments have shown safety and efficacy in various clinical trials, cementing Anika’s reputation for addressing orthopedic concerns.
Conclusion
Anika Therapeutics Inc. produces Orthovisc knee injections, demonstrating the company’s commitment to quality and innovation. The Orthovisc manufacturer adheres to stringent regulatory compliance standards, incorporates advanced manufacturing facilities and production methods, and ensures the delivery of a high-quality product for knee osteoarthritis (OA) management.
The medical technology company has ongoing research initiatives and innovations, further highlighting its dedication to advancing orthopedic medicine. Orthovisc, Monovisc, and the recent Orthovisc-T have underscored Anika’s efforts to take significant steps to improve patient’s quality of life. These insights into Orthovisc’s product development provide the manufacturer’s dedication to quality, safety, and innovation.
FAQs
1) What is Orthovisc, and how does it work?
Orthovisc is a non-surgical solution for knee osteoarthritis (OA) conditions. It supplements the lost HA in the affected knee joint. When injected, Orthovisc acts as a lubricant and shock absorber for the joints, allowing pain-free movement and improved function and mobility.
2) How effective is Orthovisc in treating knee osteoarthritis?
Orthovisc’s high molecular weight hyaluronan composition indicates safety and effectiveness in treating mild to severe cases of knee osteoarthritis. Studies have shown that Orthovisc provides significant pain relief to patients.
3) How is Orthovisc manufactured, and what quality control measures are in place?
Anika Therapeutics Inc. manufactures Orthovisc, which involves creating a sterile, non-pyrogenic, viscoelastic solution in a single-use syringe. They utilize advanced technology to build products more efficiently, safely, and with higher quality. Orthovisc knee injections involve quality control measures, including monitoring, evaluating, and maintaining the quality of the medicine.
4) When should healthcare professionals suggest Orthovisc knee injections?
Healthcare professionals may suggest Orthovisc knee injections when conservative therapies have not adequately relieved patients. This therapy typically recommends a series of three to four injections, given once weekly.
References
- Neustadt, D., Caldwell, J., Bell, M., Wade, J., & Gimbel, J. (2005). Clinical Effects of Intraarticular Injection of High Molecular Weight Hyaluronan (Orthovisc®) in Osteoarthritis of the Knee: A Randomized, Controlled, Multicenter Trial. The Journal of Rheumatology 2005; 32:10; The Journal of Rheumatology. https://www.jrheum.org/content/jrheum/32/10/1928.full.pdf
- Therapeutic Product Innovation & Performance – Anika Therapeutics. (2022, January 10). Anika.com. https://anika.com/about-anika/